NABL Accreditation (ISO 17025)

What is Accreditation ?
Laboratory accreditation is a procedure by which an authoritative body gives formal recognition of technical competence for specific tests/ measurements, based on third party assessment and following international standard.
Confidence in accreditation is obtained by a transparent system of control over the accredited laboratories and an assurance given by the accreditation body that the accredited laboratory constantly fulfils the accreditation criteria.
Accredited laboratories can objectively state conformance of product or service to specified requirements. It is important for the purchaser, regulator, government, and the public to be able to identify accredited testing and calibration laboratories.
Why Accreditation ?
• Confidence – it has been done right
• Competence – get the right answer
• Equivalence – get the same answer
• Independence – nothing else is going on
• Appropriateness – fit for purpose
• Repeatability – get the same answer twice
• Reproducibility – others get same answer
Benefits of Accreditation
Formal recognition of competence of a laboratory by an Accreditation body in accordance with international criteria has many advantages :
Increase of confidence in Testing/ Calibration data and of personnel performing work.
Better control of laboratory operations and feedback to laboratories as to whether they have sound Quality Assurance System and are technically competent.
Potential increase in business due to enhanced customer confidence and satisfaction
Customers can search and identify the laboratories accredited by NABL for their specific requirements, from the NABL Web-site or Directory of Accredited Laboratories.
Users of accredited laboratories will enjoy greater access for their services, in both domestic and international markets, when tested by accredited laboratories
Savings in terms of time and money due to reduction or elimination of the need for re-testing.
“laboratory accreditation provides a ready means for customers to find reliable testing and calibration services.”
About NABL
National Accreditation Board for Testing and Calibration Laboratories (NABL) is an autonomous body under the aegis of Department of Science & Technology, Government of India, and is registered under the Societies Act.
NABL has been established with the objective to provide Government, Industry Associations and Industry in general with a scheme for third-party assessment of the quality and technical competence of testing and calibration laboratories. Government of India has authorised NABL as the sole accreditation body for Testing and Calibration laboratories.
In order to achieve this objective, NABL provides laboratory accreditation services to laboratories that are performing tests/ calibrations in accordance with ISO/ IEC 17025 General Requirements for the Competence of Testing and Calibration Laboratories and ISO/IEC 15189 ‘Medical laboratories – Particular requirements for quality and competence’.
These services are offered in a un-biased manner and are accessible to all testing and calibration laboratories in India and abroad, regardless of their ownership, legal status, size and degree of independence
International Linkages
NABL maintains its linkages with the international bodies like International Laboratory Accreditation Co-operation (ILAC) and Asia Pacific Laboratory Accreditation Co-operation (APLAC). NABL is a full member of both and regularly takes part in the Technical Committee Meetings of both ILAC & APLAC, engaged upon development and updating of guidelines connected with accreditation activities.
In order to achieve the objective of the acceptance of test/ calibration data across the national borders, NABL operates and is committed to update its laboratory accreditation system as per international norms.
NABL Accreditation Scope
Testing Laboratories (Based on ISO / IEC 17025)
– Biological
– Chemical
– Electrical
– Electronics
– Fluid-Flow
– Mechanical
– Non-Destructive Testing
– Photometry
– Radiological
– Thermal
– Forensic
– Clinical
Clinical Laboratories (Based on ISO/IEC 15189)
– Clinical Biochemistry
– Clinical Pathology
– Haematology and Immunology
– Microbiology and Serology
– Histopathology
– Cytopathology
– Cytogenetics
– Nuclear Medicine
Calibration Laboratories (Based on ISO / IEC 17025)
– Electro-Technical
– Mechanical
– Fluid flow
– Thermal & Optical
– Radiological
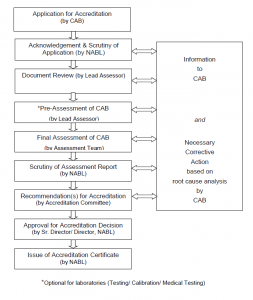
Accreditation Procedure
To know more about NABL Accrediations and NABL Consultancy Services please contact us on +91 9600001996 and mail to info@nucleus-india.com
